8 Jan 2021
Glaucoma diagnosis and management
Leila Bedos Senon DVM, MRCVS describes the manifestations of this issue, its classifications and causes, and medical and maintenance options for each.

Aqueous humour (AH) is a transparent fluid located in the anterior chamber, posterior chamber and iris. It is constantly produced in the processes of the ciliary body by epithelial cells by active secretion, ultrafiltration and simple diffusion1.
AH enters the posterior chamber, passes through the pupil and exits the eye through the iridocorneal angle, between the pectinate ligaments and into the ciliary cleft.
Two AH outflow pathways exist:
- The conventional pathway, which is the major outflow channel in the dog and accounts for at least 85% of the aqueous humour exit.
- A second site for aqueous humour exit is the uveoscleral outflow. This accounts for approximately 15% of the total aqueous humour outflow in the dog. This is the unconventional pathway2,3.
In theory, glaucoma could result from two possible mechanisms:
- increased output
- a decreased outflow of aqueous humour
Glaucoma is a related group of clinical syndromes that are characterised by elevated intraocular pressure (IOP), optic nerve damage and blindness4. It is defined as an elevated IOP secondary to a decreased exit of aqueous humour from the eye. The consequence is a progressive loss of vision due to deterioration of the neurosensory tunic of the eye4.
Glaucoma is a common disease in dogs – and despite more than two decades of constant research, it still remains a common cause of blindness in dogs.
Elevated IOP creates tension on all ocular structures2. The globe itself expands in response to the elevated pressure; however, the total enlargement is most apparent in young animals as the scleral wall is thin.

With prolonged elevated IOP, lens opacities like cataract formation has been seen in glaucoma due to an alteration on the lens nutrition2 and lens zonule breakage with secondary lens luxation are frequently secondary to buphthalmos8.
Confusion exists in the literature as to the classification of glaucomas in the dog. Therefore, the following categories have been established:
- Congenital glaucoma – the dog is born with an elevated IOP or evidence of previous elevated IOP – for example, buphthalmos3 (Figure 1).
- Primary glaucoma – no pre‑existing ocular disease and goniodysgenesis is present (the exception to the goniodysgenesis is the primary open-angle glaucoma in beagles)9,10.
- Secondary glaucoma – pre‑existing disease in the eye that predisposes elevated IOP11. Examples of this are uveitis, peripheral anterior synechiae, pupillary block causing iris bombe and luxated lenses.
Clinical manifestations of glaucoma
Acute manifestations
Acute manifestations include:
- red eye secondary to congestion of episcleral and conjunctival vessels8
- corneal oedema due to pressure‑induced impairment of endothelial pump function8
- ocular pain, seen by blepharospasm or general discomfort12
- fixed and dilated pupils due to pressure-induced paralysis of the iris sphincter muscle8
Chronic manifestations
Chronic manifestations include:
- enlargement of the globe (buphthalmos) due to pressure‑induced globe stretching8
- corneal striae (breaks in Descemet’s membrane) due to globe stretching8
- lens subluxation or luxation as a consequence of breakdown of lens zonules as the globe enlarges8
- degeneration of the retina that manifests by hyper-reflectivity of the tapetum and vascular attenuation7
- degeneration of the optic nerve manifested by optic nerve cupping, a loss of optic nerve axons resulting in a small, dark-coloured, cupped (recessed) optic disc8
Diagnosis of the different types of glaucoma
Congenital glaucomas
Diagnosis of congenital glaucoma in the puppy warrants a complete ophthalmic examination, including gonioscopy of all the littermates.
All eviscerated or enucleated eyes should be fixed and submitted for ocular histology to confirm the cause of the glaucoma13.
The common methods of therapy are enucleation or laser ciliary ablation. Evisceration and implantation of a silicone prosthesis is the preferred form of therapy if buphthalmos is mild.
Primary glaucomas
Primary glaucomas are usually associated with an abnormally developed iridocorneal angle or goniodysgenesis; a failure in rarefaction of the iridocorneal angle mesoderm to form normal pectinate ligaments10. These glaucomas have a bilateral potential and are considered to be inherited3.
Primary glaucoma usually presents in middle-aged, pure‑bred dogs with an elevated IOP without any recognised intraocular disease.
The pathogenesis of primary glaucoma in the dog is poorly understood, but it is now known the abnormality can progress with age and most likely develop clinical signs of glaucoma (fewer than 10% will develop bilateral glaucoma in late life) – meaning the presence of goniodysgenesis does not automatically indicate that primary glaucoma will develop8,14.
It is likely the cause of the glaucoma also involves abnormalities in the development of the physiological functions involved in the outflow of aqueous humour.
Some forms of primary glaucoma exist that occur with anatomically normal appearing iridocorneal angles (called open-angle glaucoma)9.
A large number of breeds are predisposed to primary glaucoma. These include the American cocker spaniel, basset hound, beagle, bouvier des Flandres, Dandie Dinmont terrier, English springer spaniel, Irish setter, toy poodle, Siberian husky, Alaskan malamute, Samoyed, chow chow and American eskimo15.
The diagnosis of primary glaucoma is based on tonometry, gonioscopy and a complete ophthalmic examination, which shows no evidence of antecedent ocular disease15.
The treatment of primary glaucoma is based on the stage of the disease (acute versus chronic) and the presence of vision. These cases are very difficult to treat successfully, and referral to a veterinary ophthalmologist is strongly recommended.
Therapeutic approaches used for primary glaucoma include:
- emergency medical management
- medical management and prophylaxis of the contralateral eye
- surgical therapy
Emergency medical management
Emergency treatment has traditionally been hyperosmotic therapy with mannitol 1g/kg to 2g/kg administered IV over 20 minutes.
This therapy will draw water from the vitreous and reduce the IOP quickly, but temporarily. Therefore, additional, longer‑acting medical therapies – such as prostaglandin analogues and carbonic anhydrase inhibitors – should be initiated at the same time as mannitol16.
Prostaglandin analogues are newer topical antiglaucoma medications that are very potent, and can reduce the IOP as quickly and effectively as mannitol in most cases. However, these drugs cause marked miosis, which may exacerbate certain types of secondary glaucoma; therefore, attention should be taken not to use these in cases related to anterior lens luxation or severe uveitis17.
Emergency therapy is required for acute onset primary glaucomas to decrease the IOP, and prevent the ongoing retinal and optic nerve degenerations. It is possible that once the IOP is back to normal, vision returns. Ongoing medical management is required in these cases. The goal of the therapy is maintenance of vision and IOP.
Medical management and maintenance therapy
Primary glaucoma is a bilateral disease, although it usually presents with one eye affected initially.
The most commonly used medications for prophylaxis are 0.5% timolol maleate every 12 hours, or a topical carbonic anhydrase inhibitor every 12 to 24 hours.
It is important to note that in primary glaucoma, the response to medical management is variable and usually short-lived. Referral to a veterinary ophthalmologist is warranted in all cases to confirm the diagnosis and direct ongoing therapy, which may include surgery.
Topical prostaglandin analogues (latanoprost, travaprost) have been found to increase uveoscleral outflow by activating the prostaglandin F receptors, which leads to widening of the ciliary body muscle and decompression of the tissue‑filled spaces along the ciliary muscle bundles. These are typically used every 12 to 24 hours1,18.
Topical carbonic anhydrase inhibitors (dorzolamide, brinzolamide) decrease the production of aqueous humour. They result in moderate IOP reduction and are used every eight hours18.
Systemic carbonic anhydrase inhibitors (methazolamide, diclofenamide and acetazolamide) reduce aqueous humour production similarly to the topical carbonic anhydrase inhibitors. They have several systemic side effects (special attention should be paid to possible increase in diuresis, gastrointestinal disturbances and increased respiratory rate due to metabolic acidosis, which are avoided with the topical drugs). Methazolamide is given 5mg/kg to 10mg/kg orally every 8 to 12 hours18.
Topical beta-adrenergic antagonists (0.5% timolol maleate) decrease aqueous humour formation. However, they cause only a mild reduction in IOP and, therefore, should not be used as a sole treatment. They are administered every 8 to 12 hours18.
Other topical and oral medications exist that are not used as commonly since the development of the topical carbonic anhydrase inhibitors and prostaglandin analogues. These are:
Topical cholinergic agents (pilocarpine, demecarium bromide or phospholine iodide) – these drugs cause increased drainage of aqueous humour out the filtration angle. They also cause miosis, painful ciliary muscle contraction and breakdown of the blood ocular barrier (uveitis)20. They are mostly avoided due to the adverse effects.
- Topical sympathomimetics (1% to 2% epinephrine; 0.5% apraclonidine) – these are thought to reduce aqueous humour production and increase the outflow18.
Surgical therapy
Three basic categories of surgical therapy exist:
- those that improve the outflow of aqueous humour (anterior chamber implants)19
- those that decrease the rate of formation of aqueous humour (laser photocoagulation, cyclocryotherapy, IV gentamicin injections)20
- those that remove the eye, such as enucleation and evisceration (for blind and painful eyes)21
Secondary glaucomas
As with primary glaucoma, the pathophysiology of secondary glaucoma is related to impairment of aqueous humour outflow. This occurs at one of two anatomical locations – the iridocorneal angle, or the pupil11.
In certain forms of secondary glaucoma, an obvious cause of the elevation of IOP is present on ocular examination, but in others it may not be obvious.
A diagnosis of secondary glaucoma requires a thorough ophthalmic examination, gonioscopy and tonometry. These glaucomas commonly present with signs of severe uveitis – for example, hyphaema or aqueous flare – and ultrasound, or CT or MRI examination may be required early for an accurate aetiological diagnosis.
Unfortunately, the clinical signs of secondary glaucoma are often very similar to the signs of primary glaucoma, and may include corneal oedema, dilated or constricted pupils, moderate to severe aqueous flare, hyphaema, anterior or posterior synechiae, anterior or posterior luxated lenses, and buphthalmos (Figures 2 and 3).
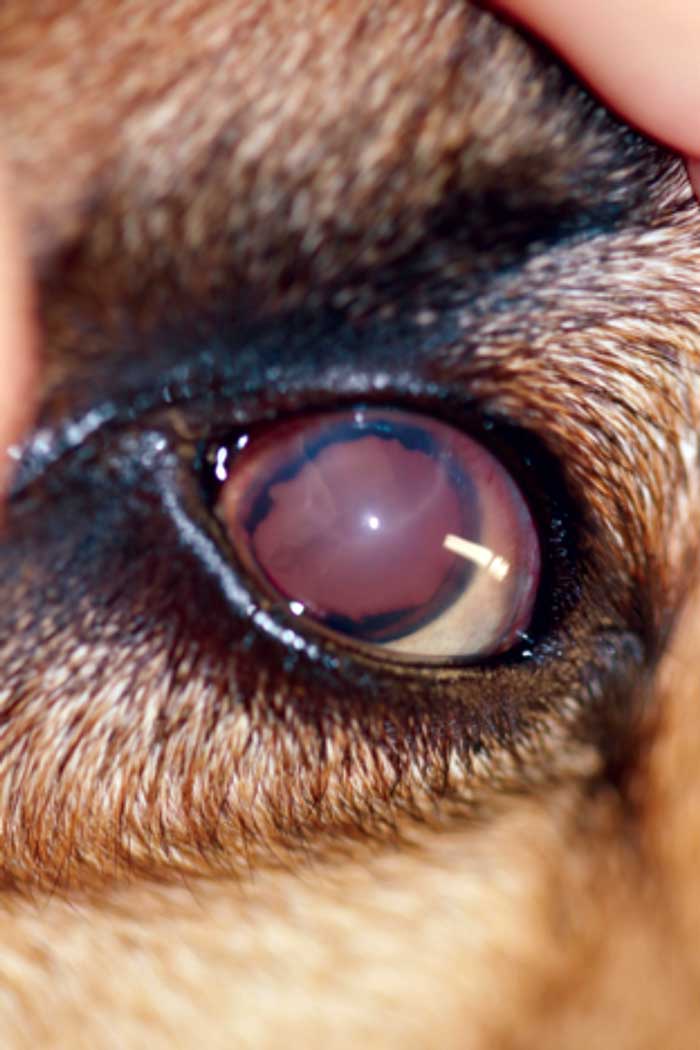
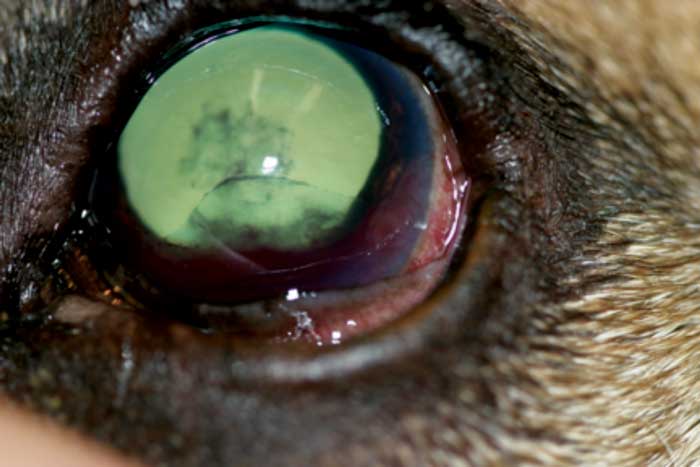
Figures 2 and 3. A small, raised tan mass was noted between the iris and corneal endothelium at approximately three o’clock to approximately five o’clock. The left anterior chamber contained a moderate amount of hyphaema and fibrin, and a fibrin clot was present adherent to the anterior lens capsule. Image © University of Saskatchewan Western College of Veterinary Medicine.
The treatment of secondary glaucoma is extremely varied and is dependent on the aetiology. It is important to stress that secondary glaucomas, like primary glaucomas, are referable diseases, and the type of therapy is based on the aetiology and presence of vision at the time of diagnosis.
Glaucomas occurring secondary to anterior lens luxations are treated by referral to an ophthalmic surgeon for removal of the lens11. Glaucomas secondary to severe uveitis are usually treated with combinations of topical anti‑inflammatory medications, including corticosteroids and NSAIDs, as well as mydriatics11.
Blind eyes with secondary glaucoma may be treated by enucleation. If enucleation is completed, the eye should be submitted for ocular histology to confirm the diagnosis. It is not uncommon to have primary glaucomas diagnosed histologically in contradiction to a clinical diagnosis of secondary glaucoma.
Evisceration and a silicone prosthesis may be implanted as a form of therapy for secondary glaucoma, with the exception of those caused by intraocular neoplasia and panophthalmitis. Due to the difficulty in diagnosis of intraocular neoplasia, it is necessary that the eye is submitted for histological diagnosis.
Important points for secondary glaucoma
Like primary glaucoma, secondary glaucoma is a frustrating disease to treat and diagnose, and they should be considered for referral. If referral is not possible, complete an ophthalmic examination.
Ultrasound is required on all eyes that cannot be completely examined due to cloudy structures.
Medical therapy will give guarded to poor results. Enucleation still remains the treatment of choice for blind buphthalmic globes unless successful medical management or an intraocular prosthesis can be implanted.
The enucleated globe should always be submitted for histopathological examination.
Referral guidelines for glaucoma
Glaucoma is a challenging disease, and all dogs with glaucoma would likely benefit from referral to a veterinary ophthalmologist. When referral is not possible, all vets are encouraged to consult with a veterinary ophthalmologist to determine appropriate therapy for the condition.
- Some drugs in this article are used under the cascade.
