8 Feb 2016
Anaesthesia and induction in neonatal companion animals

Figure 4. Puppy in incubator after premedication.
This article initially discusses what a neonate is and the differences between neonates and adults to take into consideration when anaesthetising neonatal patients, including anatomical differences (cardiovascular, respiratory, renal, hepatic, nervous and thermoregulatory systems) and pharmacological differences (considering drug metabolism, protein binding, body surface area, body fat/water content, the blood-brain barrier and the nervous system).
Preoperative management is then discussed, including problems with IV access (placement of intraosseous catheters is described), regulating body temperature, approach to feeding and what premedications can be used. The anaesthetic procedure is described, including induction of anaesthesia, maintenance of anaesthesia, fluid therapy, temperature control and monitoring during anaesthesia, with tips on how to prevent common problems.
Patient management through the postoperative period is also discussed – particularly the importance
of continuing to warm patients, returning to a normal feeding pattern as soon as possible or the need to supplement feeding, if necessary, ongoing fluid therapy and postoperative drug requirements.
A neonate is considered to be a puppy or kitten younger than four weeks. This article will discuss what needs to be considered when preparing to anaesthetise a neonatal patient, how it may be undertaken and postoperative care.
Differences between neonates and adults
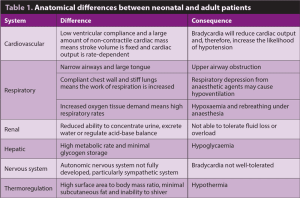
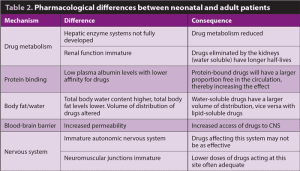
Many anatomical, physiological and pharmacological differences exist between neonatal and adult patients. Those that are important to consider with regards to anaesthetising neonatal patients are summarised in Tables 1 and 2.
What is needed to prepare preoperatively
Intravenous access
Gaining intravenous access can be challenging and occasionally the patient may need to be sedated or even anaesthetised (via mask) to allow this. Application of an eutectic mixture of local anaesthetic cream on the catheter site may be helpful. This should ideally be covered with a waterproof dressing and 30 minutes to 60 minutes should be allowed for full effect.
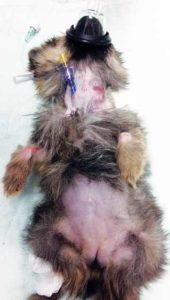
The 5% cream contains 25mg/g of both lidocaine and prilocaine, so care should be taken with dosing, although there is no known systemic uptake in veterinary patients. If a premedication is given, waiting for it to take full effect before attempting to place a catheter is helpful. In the most challenging patients, the jugular vein could be cannulated (Figures 1 and 2) or a short-term intraosseous catheter placed.

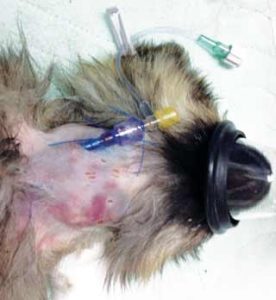
Intraosseous catheters can be used in a similar fashion to intravenous catheters and are relatively simple to place in the proximal humerus, proximal femur or proximomedial tibia (Figure 3). Unless the patient is collapsed, it is probable an intraosseous catheter will need to be placed under sedation/anaesthesia. A hypodermic needle (18 gauge to 25 gauge) can be used, the area over site of insertion should be clipped and prepared aseptically and local anaesthetic can be injected (care with toxicity).
Neonatal bones are relatively soft, so the needle should pass through the cortex fairly easily using a twisting action. To confirm correct positioning, movement of the needle should result in movement of the limb. A T-connector or giving set can be attached, the catheter secured to the skin with sutures or tape and then a dressing applied.
Intraosseous catheters should be maintained in a similar fashion to intravenous catheters.
Feeding
Neonatal patients do not need to be starved preoperatively as they are at risk of hypoglycaemia. Ideally, blood glucose should be monitored perioperatively and supplemented if necessary.
Body temperature

Neonates are prone to hypothermia, so warming is important – particularly if they are receiving a premedication. Use of coats/blankets/heated beds or an incubator (Figure 4) is helpful. If possible, pre-warming should be taken into consideration.
Weight
Using small scales to accurately weigh a patient provides an initial point of reference and helps prevent over-dosage.
Pre-anaesthetic medication
Pre-anaesthetic medication allows for reduction in the dose of (often less cardiovascularly stable) anaesthetic induction/maintenance agents and helps minimise the stress involved with handling neonates. Incorporation of an analgesic is also important to prevent hyperalgesia in young patients.
Opioids
Opioids provide analgesia and may provide enough sedation in neonatal patients alone. However, they can lead to bradycardia (leading to decreased cardiac output and, therefore, hypotension) and respiratory depression, so use as low a dose as possible. Short-acting agents (for example, pethidine or fentanyl) are useful (Table 3).
Sedatives

Benzodiazepines are more likely to cause sedation than in adults, but may still cause paradoxical excitement. There is minimal effect on the cardiovascular system and good skeletal muscle relaxation, but respiratory depression is a risk (particularly when combined with other drugs). They undergo hepatic metabolism, so care with repeated dosing should be taken (Table 3).
Acepromazine can cause profound sedation in neonates and, due to its peripheral vasodilatory effects, can compromise the cardiovascular system. It has a long duration of action and is metabolised in the liver, so should be avoided unless necessary. Alpha-2 agonists cause bradycardia and respiratory depression, and are metabolised by the liver, so should also be avoided. However, many of their effects can be antagonised, which can be an advantage.
NSAIDs
There is no evidence to support safe NSAID use in animals below six weeks old.
Emergency drugs
Be prepared to use anticholinergics to maintain the heart rate in neonates (although, because of the immature sympathetic nervous system, it is questionable how effective they are in animals below two weeks old).
Anticholinergic drugs may decrease the amount of respiratory secretions, but increase the viscosity, so airway obstruction is a risk. Atropine and glycopyrronium are most commonly used to treat bradyarrhythmias; however, glycopyrronium has a longer duration of action and is less likely to cause sinus tachycardia.
Induction and maintenance of anaesthesia
Pre-oxygenation
Pre-oxygenation is helpful due to high tissue oxygen demand and potential for respiratory depression. It will increase the available window for endotracheal intubation.
It is important to make this as stress-free as possible to minimise increases in oxygen demand.
Induction of anaesthesia
Intravenous agents
If an intravenous catheter has been placed, induction with an intravenous agent is suitable. Propofol (1mg/kg to 4mg/kg
premedicated, 6mg/kg to 7mg/kg unpremedicated) can be used for induction of anaesthesia, but as it is highly lipid soluble, highly protein-bound and requires some hepatic metabolism (although it also undergoes extrahepatic metabolism) it should be administered slowly to effect.
Alfaxalone (2mg/kg to 5mg/kg) has been reported to be suitable for induction of anaesthesia in puppies and kittens younger than 12 weeks, although is not licensed at this age.
Again, it should be administered slowly to effect. Co-administration of a benzodiazepine at the time of induction may reduce the amount of induction agent required.
Volatile agents
Induction of anaesthesia with a volatile agent (for example, isoflurane or sevoflurane) can be considered if intravenous access has not been obtained, and heavy sedation is contra-indicated. Use of a well fitting mask is important and will help reduce environmental contamination; take care to ensure carbon dioxide can be eliminated.
Sevoflurane is often better tolerated by patients than isoflurane as its odour is less pungent and it does not appear to irritate mucous membranes. Sevoflurane is less soluble in blood compared to isoflurane, leading to quicker induction of, and recovery from, anaesthesia, although its decreased solubility is accompanied by a decrease in potency, meaning higher vaporiser settings are required (up to 6% to 8% for induction in unpremedicated patients).
Anaesthetic induction with a volatile agent may be less preferable in patients with dyspnoea or at risk of regurgitation/aspiration. In some cases the excitement phase of induction of anaesthesia can be seen.
Endotracheal intubation
Rapid endotracheal intubation is preferred – especially when intravenous agents are used, so be prepared with a laryngoscope and a variety of sizes of endotracheal (ET) tubes. Also, consider having some urinary or intravenous catheters ready to use instead to provide oxygen (the ET tube connector for a 3.5mm ET tube should connect to the end of a catheter), or a well fitted mask.
Lidocaine hydrochloride can be used as the larynx of neonates is fragile (each spray contains approximately 2mg to 4mg of lidocaine). Suction for the upper airway should also be available.
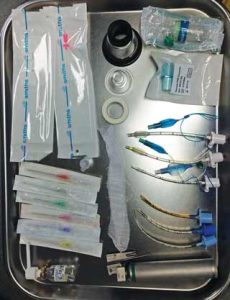
Figures 5 and 6 show examples of equipment prepared for induction of anaesthesia.
Maintenance of anaesthesia
Oxygen should always be provided, even for short minor procedures under sedation. Non-rebreathing anaesthetic systems have less resistance, so commonly a T-piece is used – for example, a Jackson Rees modified T-piece with adjustable pressure limiting valve – as it will also allow for intermittent positive pressure ventilation if required. High fresh gas flow rates (2.5 to 3 times minute volume) will be needed to avoid rebreathing; however, this will increase the risk of hypothermia.
Reducing dead space is important – low dead space ET tube connectors are available and ET tubes can be cut down if too long. Using volatile agents to maintain anaesthesia is preferred to intravenous agents (which are more likely to accumulate due to decreased drug metabolism/elimination), but care should also be taken with inhalants as both isoflurane and sevoflurane can cause dose-dependent respiratory and cardiovascular depression.
Intravenous fluid therapy
Intravenous fluid therapy is required to replace any losses and provide haemodynamic support.
Neonates cannot tolerate high rates of administration because of the lack of compliance in the neonatal myocardium, so high fluid rates/fluid boluses should be avoided in normovolaemic patients. However, neonates do not tolerate fluid loss well, therefore blood loss needs to be monitored closely and compensated for with an increased fluid rate if necessary.
Generally, isotonic crystalloid fluids are used – for example, lactated Ringer’s solution. Ideally, use narrow bore giving sets with syringe drivers or an infusion pump, or flow regulator sets/burettes and take care with volumes/air bubbles when flushing. Fluids should be warmed or at room temperature before administration.
Subcutaneous or intraperitoneal fluid administration is only useful for maintenance fluid therapy. The intraosseous route can be used if intravenous access has not been obtained.
Temperature control
Neonates have a higher surface area to volume ratio, making them more susceptible to hypothermia.
Prevention is always better than cure, so warming the patient from the start of the anaesthetic period (from or even before premedication) will give the best results.
Heat and moisture exchange devices, fluid warmers, heated beds, warm air devices and bubble wrap/blankets (with a surgical window cut in if necessary, or wrapped around extremities) can all be used.
Blood glucose
Checking blood glucose at induction of anaesthesia and throughout the procedure can guide whether the use of glucose supplemented fluids (2.5% to 5%) is required.
Monitoring
The attention of the anaesthetist is the best monitor; however, access to the patient may be limited. ECG pads pre-trimmed to fit (or use of contact gel rather than spirit with ECG clips to help preserve temperature) will help monitor cardiac activity. Alternatively, an oesophageal stethoscope could be used to monitor heart rate.
Capnography can be used to aid the monitoring of ventilation and cardiac output. There may be some inaccuracy because of the small tidal volume of neonatal patients when side-stream monitoring is used.
Mainstream monitors will be more accurate, but the size of the sampling unit may increase dead space and produce some physical drag. Blood pressure measurement can be difficult due to problems with cuff sizes; using a Doppler flow probe may be more reliable than oscillometric measurement, but will only provide systolic pressure.

Blood pressure in neonates is lower than in adults (Table 4); however, if a patient is deemed hypotensive and does not respond to a reduction in anaesthetic agent or increase in fluid rate, assess what the heart rate is and treat as appropriate. For example, if the heart rate is low, use of an anticholinergic may improve heart rate and, therefore, blood pressure. If the heart rate is normal or high, consider the use of pressors and inotropes (Table 3). Pulse oximetry should be used (take care with the probes compressing tissue too much) and body temperature should be monitored.
Local anaesthetic
Consider using local anaesthetic to reduce the amount of anaesthetic agents required, and potentially reduce the amount of postoperative analgesia. Diluting the required dose with saline by 50% will give a greater volume to use and help prevent overdosing.
Managing patients postoperatively
Tracheal extubation
It is important to have suction available to clear airway obstructions and extubate the trachea as late as possible to allow oxygen supplementation.
Ideally, oxygen should be provided until the patient is fully recovered – this can be via flow by or an oxygen-enriched environment (for example, oxygen cage/tent or incubator).
Temperature
Using an incubator is ideal, allowing for simultaneous warming, oxygen supplementation and observation. Excess surgical prep should be removed and the patient dried as soon as possible to prevent further loss.
Warming aids should be used for as long as possible, even after recovery, to prevent heat loss while the patient is inactive in recovery. Avoid overheating.
Feeding
A quick recovery will allow the patient to eat as soon as possible after anaesthesia. If the patient is taking a long time to recover, consider checking blood glucose.
Most neonates will still be suckling from the dam, so need to be returned to her as soon as possible. If necessary, feeding may need to be supplemented with a milk replacer (see Table 4 for daily calorie requirements), including the use of gastric tubes, until the patient is able to suckle.
Fluids
Maintenance rates should be continued until the patient is eating/drinking adequately. Weighing the patient twice daily can help tracking of any weight changes.
Drugs
Analgesia will be required postoperatively if the patient has undergone a painful procedure, and can help avoidance of inappetence and abnormal behaviour (although this can be difficult to interpret in the neonate).
Opioids can be used; however, caution should be used with doses to prevent excessive sedation, respiratory depression
and inappetence.
- Drugs mentioned in this article are not licensed for use in neonatal patients.
Latest news

Small animal
Is faecal calprotectin the missing link in your inflammatory GI cases?
Sponsored
16 Jun 2025